Cosmetic Surgery Blog
The Ugly Duckling Sign

“The Ugly Duckling Sign” may be the best way for none dermatologist to recognize a melanoma.
Most patient education materials talk about using the A,B,C,D, Es of melanoma detection. (Asymmetry, Boarder irregularity, Color variation, Diameter larger than 6mm and Evolution or change). A recent study suggests that for most patients looking for the “ugly duckling” among a group of moles may be a more effective self screening technique. That doesn’t mean you should ignore the A,B,C,D,Es, but you should also look out for the ugly duckling, before it becomes an even uglier duck!
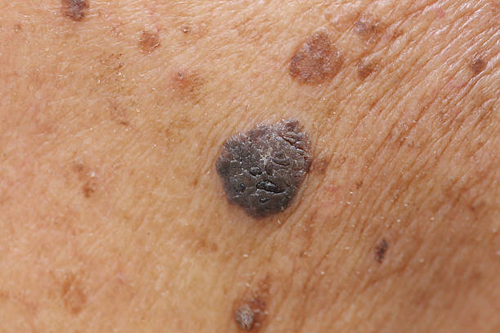
Link to abstract:
http://www.jaad.org/article/S0190-9622(17)32054-6/abstract
Citation:
Journal of the American Academy of Dermatology
The Role of the Ugly Duckling Sign in Patient Education
J Am Acad Dermatol 2017 Sep 27;[EPub Ahead of Print], M Ilyas, CM Costello, N Zhang, A Sharma
Genetic Testing For Melanoma Risk: who should have it?
A recent study from France published in the Journal of the American Medical Association looked at risk factors that should lead to genetic testing for the high risk melanoma gene. Their conclusion was that testing was merited in patients with any 3 of the following:
- A melanoma under the age of 40 years old.
- Two cutaneous melanomas or a related malignancy (eg. pancreatic cancer)
- A first-degree relatives with two cutaneous melanomas or related tumor.
- A second-degree relatives with two cutaneous melanomas or related tumor.
So it seems that for the vast majority of melanoma patient this type of genetic testing may not be indicated.
Link to Abstract:
Melanomas usually just happen on their own!
The majority of melanomas arise from new lesions rather than existing moles, according to a review published online Aug. 29 in the Journal of the American Academy of Dermatology.
While we always emphasis the importance of following the appearance of your existing moles the fact is that most melanomas (70%) do not derive from existing moles “going bad” , but just develop as melanomas from the very start.
You should still follow your moles (30% of melanomas do derive from existing moles), but also know your whole skin so you can recognize any new moles. To this end we highly recommend full body digital photography as a baseline documentation of your moles and skin against which any potentially changing or new moles can be compared.
Researchers conducted a review of 38 previously published medical studies involving 20,126 melanomas.
The findings showed that only 29.1 percent of the skin cancers started in moles patients already had, while 70.9 percent arose as new lesions on the skin.
“In conclusion, in this systematic review and meta-analysis we found that less than one-third of melanomas were nevus-associated.”
Link to abstract:
http://www.jaad.org/article/S0190-9622(17)32051-0/abstract
Melanoma Rates Still Rising in California
A new study from the Journal of Investigative Dermatology has confirmed that the rates of melanoma are still on the rise among white Californians. All the more reason to protect yourself from the damaging rays of the sun, check your own skin routinely and have an annual skin cancer screening exam.
Title of article: “Continued increase in melanoma incidence across all socioeconomic status groups in California, 1998-2012”
Link to abstract:
http://www.jidonline.org/article/S0022-202X(17)31867-5/pdf
Thin Melanomas – Age Matters!

A new study suggests that in young patients with thin melanomas (0.5 -1mm depth) there may be an approximately 5% risk of lymph nodes being involved. As such, even though present guidelines do not recommend it, in young patients with melanomas between 0.5 and 1mm sentinel node biopsy may be considered.
Association Between Patient Age and Lymph Node Positivity in Thin Melanoma
Andrew J. Sinnamon, MD1; Madalyn G. Neuwirth, MD1; Pratyusha Yalamanchi, BA1; et alPhyllis Gimotty, PhD
JAMA Dermatol. Published online July 19, 2017. doi:10.1001/jamadermatol.2017.2497
Link to abstract:
http://jamanetwork.com/journals/jamadermatology/article-abstract/2643739
Mohs wins out again
Recent published study confirms Mohs micrographic surgery as the patients treatment of choice even for superficial skin cancers. Patients in the study preferred Mohs surgery not only because it gave a better long term cure rate but it was seen as a much more tolerable procedure compared to the alternative treatments.
Link to abstract:
https://insights.ovid.com/crossref?an=00042728-900000000-99021
Treatment Patterns, Outcomes, and Patient Satisfaction of Primary Epidermally Limited Nonmelanoma Skin Cancer
Dermatologic Surgery. Publish Ahead of Print():, JUN 2017
Benjamin A. Drew; Pritesh S. Karia; and 3 more
TAKE-HOME MESSAGE
-
- In this retrospective cohort study, 550 patients with superficial basal cell carcinoma (BCC) and squamous cell carcinoma in situ (SCCis) were treated with either Mohs micrographic surgery (MMS) or non-MMS (5% 5-fluorouracil, cryotherapy, ingenol mebutate, or imiquimod). Although both groups had high 5-year recurrence-free survival, this was significantly higher with MMS compared with non-MMS (99% vs 95%, respectively; P = .004). Moreover, 97% of MMS patients were willing to undergo another treatment compared with 86% of non-MMS patients (P = .024). Results from a satisfaction survey offered to 25% of patients indicated that a prolonged treatment course and pain contributed to dissatisfaction on the part of the patients who did not undergo MMS.
- This study found low recurrence rates with both surgical and nonsurgical treatments for superficial BCC and SCCis. However, MMS was associated with higher patient satisfaction and cure rates compared with non-MMS.
– InYoung Kim, MD, PhD
BACKGROUND
Epidermally limited nonmelanoma skin cancer (ELNMSC) (superficial basal cell carcinoma [SBCC] and squamous cell carcinoma in situ [SCCIS]) is common. Data on outcomes and patient satisfaction are lacking.
OBJECTIVE
To examine treatment efficacy and satisfaction in ELNMSC patients.
PATIENTS AND METHODS
Retrospective cohort study of adults with primary SBCC or SCCIS. A 25% random subset completed a satisfaction questionnaire.
RESULTS
Five hundred and fifty patients with 227 SBCC and 451 SCCIS were included; 329 tumors (49%) were treated with Mohs micrographic surgery (MMS) and 349 (51%) with non-MMS (imiquimod [n = 26], 5% 5-fluorouracil [n = 234], ingenol mebutate [n = 32], or cryotherapy [n = 57]). Five-year recurrence-free survival was high in both groups, with MMS having a small but statistically significant advantage (99% vs 95%, p = .004). More MMS patients were willing to undergo treatment again (97% vs 86%, p = .024). Dissatisfaction was mostly due to prolonged treatment course and pain associated with non-MMS treatments.
CONCLUSION
Surgical and nonsurgical treatments for primary ELNMSC have low recurrence rates, though cure rate and patient satisfaction are higher with MMS. Treatment choice for epidermal NMSC may be guided through patient preferences regarding ability to comply with topical treatment, out-of-pocket costs, desire to treat surrounding field disease, and desire to avoid a surgical scar.
Wear UV Protective Sunglasses to Protect Your Eyelids from Skin Cancer
We already know that UV protective sunglasses are important to lessen the long term risk of cataracts. Now a recent study shows that most of us do not apply sunscreen to our eyelids, leaving them susceptible to UV damage. Wearing UV protective sunglasses can patch that problem, while looking good at the same time!
Yet Another Good Reason to Quite Smoking!

A team from QIMR Berghofer Medical Research Institute studied nearly 19 thousand people and found that current smokers were significantly more likely to develop a squamous cell carcinoma (SCC) of the skin than non-smokers. “We don’t yet understand how smoking might increase the risk of SCC, but these findings strongly suggest that by quitting, smokers are lowering their risk of SCC to the same level as someone who has never smoked. This is another good reason to quit.”
Read more: http://doctor.ndtv.com/cancer/smoking-increases-risk-of-skin-cancer-1721838
Melanoma linked to Parkinson’s Disease

Patients with Parkinson’s disease are about four times more likely to develop melanoma, and conversely, patients with melanoma have a four-fold higher risk of developing Parkinson’s, according to a study published in the July issue of the Mayo Clinic Proceedings.
The new study included 974 patients with Parkinson’s disease and compared them to 2,922 individuals without the movement disorder. The study also included 1,544 with melanoma. All of the study volunteers came from one county in Minnesota.
The researchers found that, compared with controls, patients with Parkinson’s disease had a 3.8-fold increased likelihood of having preexisting melanoma, while patients with melanoma had a 4.2-fold increased risk of developing Parkinson’s disease. The investigators said that since there is such a strong connection between these diseases, doctors treating patients for either disease should watch for signs of the other. They also recommend that doctors counsel patients about their risk of the other condition.
“Future research should focus on identifying common genes, immune responses, and environmental exposures that may link these two diseases,” first author Lauren Dalvin, M.D., of the Mayo Clinic in Rochester, Minn., said in a Mayo news release. “If we can pinpoint the cause of the association between Parkinson’s disease and melanoma, we will be better able to counsel patients and families about their risk of developing one disease in the setting of the other.”
Here is the link to the abstract of the original article: http://www.mayoclinicproceedings.org/article/S0025-6196(17)30239-2/abstract
More Is Not Always Better
In the past, lymph node dissection was thought to benefit patients with melanoma. A recent study in the New England Journal of Medicine, however, has shown that it does not seem to have any added benefits. The study concluded that complete lymph node removal was no better than less extensive surgery and observation for extending survival.
Among those who participated in the study, about a quarter developed lymphedema, an unsightly swelling in an arm or leg caused by a lymphatic system blockage. Among those who only had their sentinel node removed, 6.3 percent developed lymphedema, while survival rates remained comparable.
Not only did these patients experience no change in survival rates, they also developed an unsightly condition that could have been avoided. The study supports the hypothesis that “Immediate completion lymph-node dissection was not associated with increased melanoma-specific survival among 1934 patients with data that could be evaluated in an intention-to-treat analysis or among 1755 patients”
Dr. Ralph Massey is a skilled cosmetic and skin cancer surgeon who will assess your needs and develop a safe and effective treatment plan based on your individual situation. Contact us if you’re interested in scheduling an information consultation where you can learn more about skin cancer treatments.
Click on the link below to find out more:
http://www.nejm.org/doi/full/10.1056/NEJMoa1613210
